2018年に本庶佑博士とともにノーベル医学生理学賞を受賞したジェームス・アリソン博士が、最初の臨床試験のことを語るインタビュー動画。
2015 Lasker DeBakey Clinical Medical Research Award 2015/09/07 Albert and Mary Lasker Foundation
Talks@12: Immunotherapy: An Answer to Cancer? 2017/11/07 Harvard Medical School
抗CTLA-4抗体(免疫チェックポイント阻害薬)の最初の臨床試験で効果があった患者さんのストーリー
Sharon Belvinさんのストーリー
上の動画でも登場したSharon Belvinさんのストーリー。
She’s the Answer to Cancer…and So Are You 2016/05/06 Cancer Research Institute
- Milestones in Medicine: How Immunotherapy Began in Cancer Care A look back at how immunotherapy began in oncology. (ARLENE WEINTRAUB SEPTEMBER 25, 2019 curetoday.com) Sharon Belvin was 23 and running out of options to treat her stage 4 melanoma in 2005 when her oncologist at Memorial Sloan Kettering Cancer Center in New York City offered to enter her in a clinical trial of a drug designed to empower her immune system to fight the cancer. By that point, she had progressed after several rounds of chemotherapy, plus radiosurgery to remove tumors that had spread to her brain. The drug blocked CTLA-4, a protein “checkpoint” that prevents the immune system from recognizing and attacking cancer. After just four treatments, 60% of Belvin’s tumors were gone. Within months, they had all disappeared, and she has been cancer-free ever since. In 2011, that drug, Yervoy (ipilimumab), became the first checkpoint inhibitor to be approved by the Food and Drug Administration (FDA).
- For this Nobel winner, fighting cancer began with his family (Oct 1, 2018 6:25 PM EST PBS) アリソン博士が2018年ノーベル医学生理学賞を受賞した直後のPBS NEWSのインタビュー動画(7:26)トランスクリプト付き
- Melanoma survivor’s unlikely recovery leads to lifestyle changes (Oct 1, 2017 sungazette.com) The treatment involved 90-minute infusions and injections in Belvin’s leg. … But the radiologist had never seen anything like it. He had to verify he’d grabbed the right labs. My tumors had shrunk by 60 percent in that first round.
- A Scientist’s Dream Fulfilled: Harnessing The Immune System To Fight Cancer (June 9, 2016 3:41 PM ET Heard on All Things Considered NPR) Sharon Belvin’s nightmare with cancer began in 2004, when she was just 22.
- Six Miracle Cancer Survivors (Robert Langreth Mar 2, 2009, 04:50pm forbes.com) An experimental drug helped Sharon Belvin, who was diagnosed with melanoma in her lung when she was 22 and spent two years in standard treatment. The drug is called ipilimumab, and it aims to trigger the immune system against cancer. Within four months, her lung tumors started to shrivel. By late 2006, they were gone. Today Belvin, now 27, is off all treatment.
Tom Telfordさんのストーリー
- How the Promise of Immunotherapy Is Transforming Oncology (Tom Telfordさんのストーリー WSJ ) He had surgery at Memorial Sloan Kettering Cancer Center, followed by months of chemotherapy. But the disease spread to his liver and kidneys. The diagnosis: Stage 4 melanoma, a skin cancer typically fatal within a year. “Death is not an option,” he told his doctor. Nine years later, against all odds, Mr. Telford is still alive. What saved him was an experimental immunotherapy drug—a medication that unleashes the body’s own immune system to attack cancer.
ipilimumabをFDAが承認
On 25 March, the FDA cleared ipilimumab, produced by Bristol-Myers Squibb, based in New York, to treat advanced melanoma, a particularly lethal form of skin cancer. Although the drug typically lengthens a patient’s life by only 4 months or so, in clinical trials a fraction of patients lived much longer.
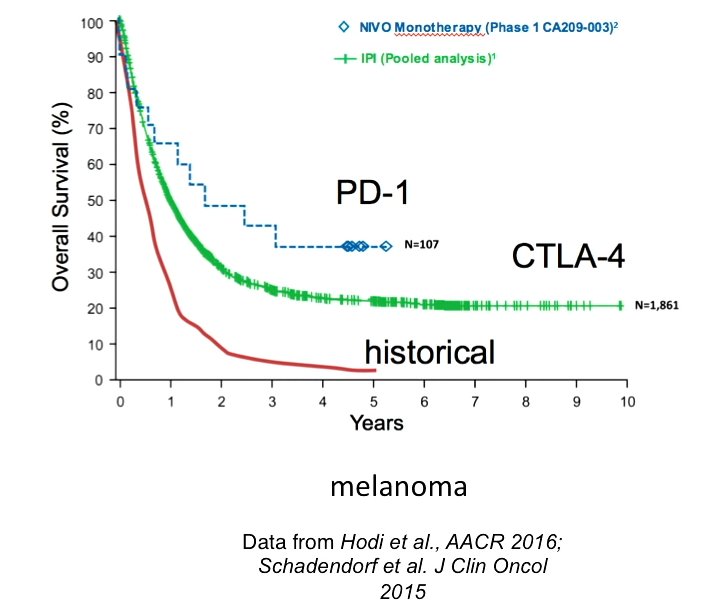
Although ipilimumab can add years of life, only 20–30% of patients show any benefit at all (F. S. Hodi et al. N. Engl. J. Med. 363, 711–723; 2010).
(引用元:Melanoma drug wins US approval. Nature volume 471, page 561 (2011) 28 March 2011)
臨床試験
- MDX-010 Antibody, MDX-1379 Melanoma Vaccine, or MDX-010/MDX-1379 Combination Treatment for Patients With Unresectable or Metastatic Melanoma (Phase 3)(NCT00094653) Study Type : Interventional (Clinical Trial) Actual Enrollment : 1783 participants Allocation: Randomized Intervention Model: Parallel Assignment Masking: Triple (Participant, Care Provider, Investigator) Primary Purpose: Treatment Official Title: A Randomized, Double-Blind, Multicenter Study Comparing MDX-010 Monotherapy, MDX-010 in Combination With a Melanoma Peptide Vaccine, and Melanoma Vaccine Monotherapy in HLA-A2*0201-Positive Patients With Previously Treated Unresectable Stage III or IV Melanoma Study Start Date : September 2004 Actual Primary Completion Date : August 2009 Actual Study Completion Date : October 2009
- IPILIMUMABの臨床試験(NIH ClinicalTrials.govデータベースの検索結果)
臨床試験の結果を報告した論文
- The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008; 8: 1. Published online 2008 Jan 17. PMCID: PMC2935787 Results from preclinical and early clinical trials support currents phase II/III testing of ipilimumab as first- and second-line therapy for metastatic melanoma.
- CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009 Feb 12;113(7):1581-8. doi: 10.1182/blood-2008-07-168468. Epub 2008 Oct 30. Twenty-nine patients with malignancies that were recurrent or progressive after allo-HCT, received ipilimumab as a single infusion at dose cohorts between 0.1 and 3.0 mg/kg. … Three patients with lymphoid malignancy developed objective disease responses following ipilimumab: complete remission (CR) in 2 patients with Hodgkin disease and partial remission (PR) in a patient with refractory mantle cell lymphoma. At the 3.0 mg/kg dose, active serum concentrations of ipilimumab were maintained for more than 30 days after a single infusion. Ipilimumab, as administered in this clinical trial, does not induce or exacerbate clinical GVHD, but may cause organ-specific IAE and regression of malignancy.
- Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. August 19, 2010 N Engl J Med 2010; 363:711-723 DOI: 10.1056/NEJMoa1003466 METHODS A total of 676 HLA-A*0201–positive patients with unresectable stage III or IV melanoma, whose disease had progressed while they were receiving therapy for metastatic disease, were randomly assigned, in a 3:1:1 ratio, to receive ipilimumab plus gp100 (403 patients), ipilimumab alone (137), or gp100 alone (136). RESULTS The median overall survival was 10.0 months among patients receiving ipilimumab plus gp100, as compared with 6.4 months among patients receiving gp100 alone (hazard ratio for death, 0.68; P<0.001). CONCLUSIONS Ipilimumab, with or without a gp100 peptide vaccine, as compared with gp100 alone, improved overall survival in patients with previously treated metastatic melanoma.
- A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naïve patients with advanced melanoma. Investigational New Drugs June 2011, Volume 29, Issue 3, pp 489–498 (Free abstract)
- Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol. 2015 Jun 10;33(17):1889-94. doi: 10.1200/JCO.2014.56.2736. Epub 2015 Feb 9.
- Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. October 5, 2017 N Engl J Med 2017; 377:1345-1356 DOI: 10.1056/NEJMoa1709684 2つのICIを併用した効果をみたもの
抗CTLA-4抗体の効果を調べた初期の論文
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996 Mar 22;271(5256):1734-6. (PDF) We reasoned that CTLA-4 blockade would remove inhibitory signals in the costimulatory pathway, resulting in enhanced rejection of the tumor cells. We injected groups of BALB/c mice …

 参照元(フルサイズの画像):
参照元(フルサイズの画像):