アクアバウンティー・テクノロジーズ社が開発した、遺伝子組換え技術により通常よりも速く成長するサケ『アクアドバンテージ・サーモン』が生産・販売され食用に供されることを、 アメリカ食品医薬品局(Food and Drug Administration; FDA)が2015年11月19日に承認しました。アクアバウンティーが最初にFDAの許可を申請したのは1996年で、実に20年近くもかかってようやくここまでたどり着いたということになります。
“The FDA has thoroughly analyzed and evaluated the data and information submitted by AquaBounty Technologies regarding AquAdvantage Salmon and determined that they have met the regulatory requirements for approval, including that food from the fish is safe to eat,” ( ‘Frankenfish:’ GMO salmon declared safe to eat, environmentalists rail against FDA. rt.com 20 Nov, 2015)
この遺伝子組換えサーモンを食べても安全というFDAのお墨付きが出たからといって、長年の論争に決着がついたとはいえないようです。「遺伝子組換え」の表示は不要というFDAの判断に不安を募らせる消費者も多く、ホールフーズマーケットやトレーダージョーズなどアメリカの大手のスーパーマーケットは、アクアドバンテージ・サーモンを取り扱わないことを以前、宣言しています。
Genetically-modified salmon approved by FDA (https://www.youtube.com/watch?v=PQSArjT8j9o)
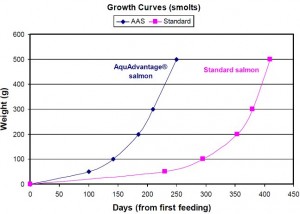
市場に出荷できるサイズにまで成長のに必要な飼育期間が半分近くにまで短縮され、コストが大きく削減できるメリットが謳われています。
「アクアドバンテージサーモン」と名づけられたこのアトランティックサーモンは、1年を通して成長ホルモンが分泌するように、遺伝子組換え技術を用いて「改良」されています。ゲンゲという別の種類の魚の不凍タンパク質(Anti-freezing protein;AFP)遺伝子の発現制御領域に、チヌークサーモン(chinook salmon)の成長ホルモンの遺伝子をつないだDNAを、アトランティックサーモン(Atlantic Salmon 学名:Salmo salar)に遺伝子導入したというものです。
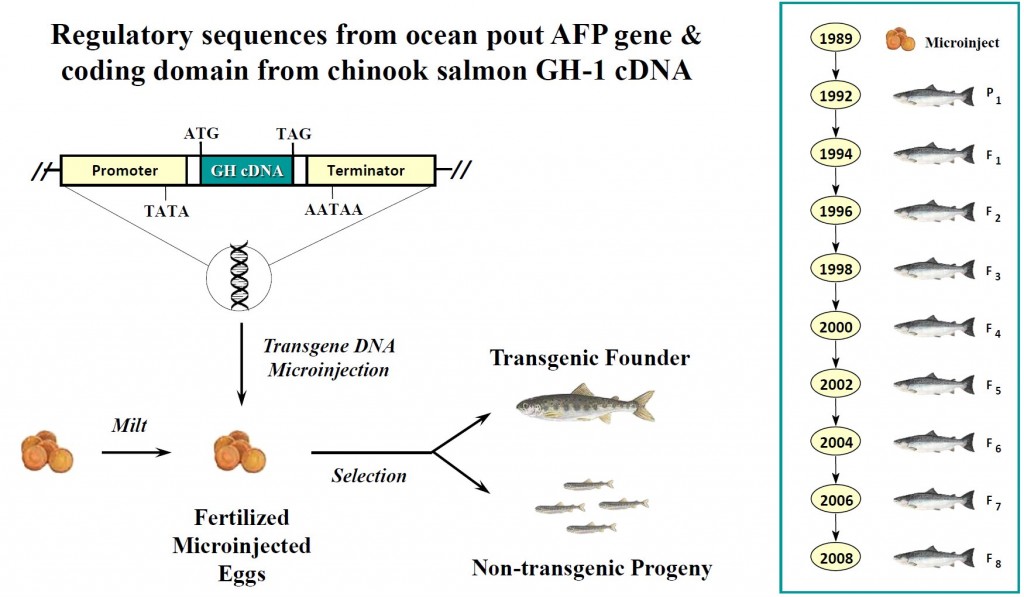 (https://www.ncbiotech.org/sites/default/files/pages/Keynote%202%20-%20AquaBounty%20Keynote%20Presentation.pdf)
(https://www.ncbiotech.org/sites/default/files/pages/Keynote%202%20-%20AquaBounty%20Keynote%20Presentation.pdf)
参考
- U.S. Food and Drug Administration (FDA) > Animal & Veterinary > Development & Approval Process > Genetic Engineering > Genetically Engineered Animals > AquAdvantage Salmon (アクアドバーンテージ・サーモンに関するFDAの文書)
- AquaBounty Technologies, Inc.
- FDA Approves AquAdvantage® Salmon (アクアバウンティ社のプレスリリースPDFファイル):”MAYNARD, Massachusetts, 19 November 2015 – AquaBounty Technologies, Inc. (AIM: ABTU; OTC: AQBT), a biotechnology company focused on enhancing productivity in aquaculture, and a majority-owned subsidiary of Intrexon Corporation (NYSE: XON), announces today that the U.S. Food and Drug Administration (FDA) has approved the Company’s New Animal Drug Application for the production, sale, and consumption of its AquAdvantage® Salmon, an Atlantic salmon that has been genetically enhanced to reach market size in less time than conventional farmed Atlantic salmon.”
- The FDA just approved genetically modified salmon for the first time. But how does it taste? (VOX Science & Health. Updated by Julia Belluz on November 19, 2015):”AquaBounty first asked the FDA to approve the fish for human consumption in 1996. But the FDA said because this was the first genetically modified animal that would be eaten by humans, the agency wanted to take it slow and weigh the pros and cons.”
- Food Fight: Policy and Politics.By R.L. Stotish, AquaBounty Technologies (PDF 28 pages)
- Genetically Engineered Salmon (April 30, 2014, Congressional Research Service)(PDF 30 pages)
- GE Salmon Faces Exclusion from Retailers (WholeFoods Magazine, December 2013):”Chains within both the natural and mainstream channels, including Whole Foods Market, Trader Joe’s and Target, have either signed on to a pledge or made clear in policy statements that they will not purchase the GE Atlantic salmon if approved.”
- The AquAdvantage Salmon Controversy – A Tale of Aquaculture, Genetically Engineered Fish and Regulatory Uncertainty. By Alain Goubau, May 11, 2011. (ハーバード大学ウェブサイト上 PDF)
-
Genetically Modified Salmon – Coming Soon? (uploaded 2011 Apr)
- Environmental Assessment for AquAdvantage® August 25, 2010. Submitted to the Center for Veterinary Medicine, US Food and Drug Administration (PDF 85 pages)
- Biotechnology in Aquaculture: Transgenics and Polyploidy. Rosalee S. Rasmussen andMichael T. Morrissey. Comprehensive Reviews in Food Science and Food Safety Volume 6, Issue 1, pages 2–16, January 2007
- Characterization and multi-generational stability of the growth hormone transgene (EO-1α) responsible for enhanced growth rates in Atlantic Salmon. Edward S. Yaskowiak, Margaret A. Shears, Alka Agarwal-Mawal, Garth L. Fletcher Transgenic Research August 2006, Volume 15, Issue 4, pp 465-480: “We have generated a stable line of growth hormone (GH) transgenic Atlantic salmon (Salmo salar) using an “all fish” gene construct (opAFP-GHc2) containing a growth hormone cDNA from chinook salmon whose expression is regulated by the 5′ promoter and 3′ termination regions derived from an ocean pout antifreeze protein (AFP) gene. In this study we show that a reorganized form of the opAFP-GHc2 construct (termed EO-1α) integrated as a single functional copy into a 35 bp repeat region of the genomic DNA. PCR based mapping revealed that the linear sequence of the EO-1α integrant was organized as follows: base pairs 1580–2193 of the ocean pout promoter region followed by the intact chinook salmon GH cDNA, the complete ocean pout antifreeze 3′ region, and the first 1678 bp of the ocean pout antifreeze 5′ region.”
- 試験研究は今 NO.045 Q&A? 最近よく耳にする3倍体魚とはどのようなものですか?:”この3倍体の特徴としては、雄は通常の2倍体と同様に成熟しますが、雌は成熟しないことが上げられます。…人工受精にはニセ雄の精子を使います。ニセ雄とは、本来雌であるものに雄のホルモンを混ぜた餌を与えることにより精子をつくれるようにしたものですが、この精子を使うと、できる子供は全部雌になります。次に、これで受精させて、およそ10分後にその受精卵に強い圧力や、温度の刺激(サケ・マスの場合は暖かい水につける)を与えると、3倍体の雌ができあがります。この受精卵に与える圧力や温度変化が強すぎると卵が死亡してしまい、逆に弱すぎると通常の2倍体になってしまいます。”
- Risk assessment and mitigation of AquAdvantage salmon (BiologyFortified. Posted by Anastasia Bodnar, Oct 16, 2010):”Pressure treatment will be used to produce triploid AquAdvantage salmon. This treatment was successful at creating 98.9% or more triploids, with 1.1% or fewer eggs remaining diploid (11). Testing of each batch of eggs will be conducted, and any batch that contains 5% or more diploids will be destroyed (7,11). Any diploid individuals are capable of reproduction, so the possibility of their escape must be controlled with other measures.”